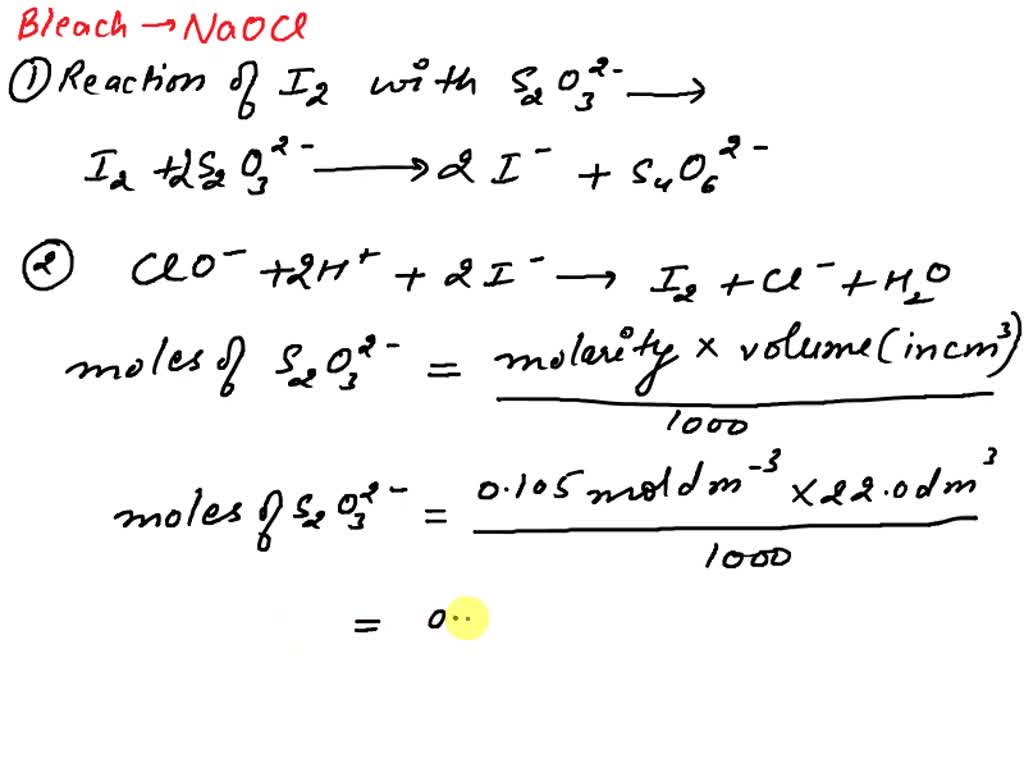Redox Titration Of Sodium Thiosulfate And Iodine . — well another redox titration with a lot molar ratio work! — mrs lucas explains the sodium thiosulfate and iodine titration theory and. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. — this redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated. — how can the reaction of iodine and thiosulfate ions be used as a titration?.
from www.numerade.com
— mrs lucas explains the sodium thiosulfate and iodine titration theory and. — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. — how can the reaction of iodine and thiosulfate ions be used as a titration?. — this redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — well another redox titration with a lot molar ratio work! redox titration of iodine molecules using aqueous sodium thiosulfate solution.
SOLVED (a) Write a balanced redox equation for the reaction between iodine and thiosulfate ion
Redox Titration Of Sodium Thiosulfate And Iodine — well another redox titration with a lot molar ratio work! — well another redox titration with a lot molar ratio work! — mrs lucas explains the sodium thiosulfate and iodine titration theory and. — this redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — how can the reaction of iodine and thiosulfate ions be used as a titration?. — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry.
From dokumen.tips
(PDF) Investigation of iodine liberation process in redox titration of potassium iodate with Redox Titration Of Sodium Thiosulfate And Iodine — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. — how can the reaction of iodine and thiosulfate ions be used as a titration?. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — this redox titration relies on the reaction between the oxidizing agent and iodide. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.slideserve.com
PPT Booklet to help with Unit 27 PowerPoint Presentation ID1951670 Redox Titration Of Sodium Thiosulfate And Iodine — this redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — how can the reaction of iodine and thiosulfate ions be used. Redox Titration Of Sodium Thiosulfate And Iodine.
From slideplayer.com
Redox titrations iodine and thiosulfate L.O. Understand how to carry out redox titrations and Redox Titration Of Sodium Thiosulfate And Iodine — this redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated. — well another redox titration with a lot molar ratio work! — how can the reaction of iodine and thiosulfate ions be used as a titration?. redox titration of iodine molecules using aqueous sodium. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.vernier.com
Potentiometric Titration of Aqueous Iodine Redox Titration Of Sodium Thiosulfate And Iodine redox titration of iodine molecules using aqueous sodium thiosulfate solution. — well another redox titration with a lot molar ratio work! — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — mrs lucas explains the sodium thiosulfate. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Redox Titration Of Sodium Thiosulfate And Iodine — mrs lucas explains the sodium thiosulfate and iodine titration theory and. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — well another redox titration with a lot molar ratio work! — how can the reaction of iodine and thiosulfate ions be used as a titration?. Note that sodium thiosulfate, na 2 s 2. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.youtube.com
Iodine / Thiosulfate Redox Titration Demonstration YouTube Redox Titration Of Sodium Thiosulfate And Iodine — this redox titration relies on the reaction between the oxidizing agent and iodide ions to produce iodine, which is then titrated. — well another redox titration with a lot molar ratio work! redox titration of iodine molecules using aqueous sodium thiosulfate solution. — how can the reaction of iodine and thiosulfate ions be used as. Redox Titration Of Sodium Thiosulfate And Iodine.
From byjus.com
Oxidation state of sulphur in sodium thiosulphate during the reaction of sodium thiosulphate and Redox Titration Of Sodium Thiosulfate And Iodine Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. — how can the reaction of. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.youtube.com
WAEC chemistry practical (Redox titration of Iodine solution, I2 /KI & Sodium thiosulfate Redox Titration Of Sodium Thiosulfate And Iodine Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — well another redox titration with a lot molar ratio work! — mrs lucas explains the sodium thiosulfate and iodine titration theory and. — this redox titration relies on the reaction between the oxidizing agent. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.youtube.com
WAEC chemistry practical (Redox titration of Iodine solution, I2/KI and Sodium thiosulfate Redox Titration Of Sodium Thiosulfate And Iodine — how can the reaction of iodine and thiosulfate ions be used as a titration?. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — well another redox titration with a lot molar ratio work! redox titration of iodine molecules using aqueous sodium thiosulfate solution. — mrs lucas explains the sodium thiosulfate and iodine. Redox Titration Of Sodium Thiosulfate And Iodine.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration Of Sodium Thiosulfate And Iodine — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. — well another redox titration with a lot molar ratio work! Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — this redox titration relies. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.youtube.com
Eureka 2017 Iodometry Redox Titration KIO3 Vs Thiosulfate YouTube Redox Titration Of Sodium Thiosulfate And Iodine redox titration of iodine molecules using aqueous sodium thiosulfate solution. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — this redox titration relies on. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.sciencephoto.com
Iodine and thiosulfate ions titration Stock Image C033/2858 Science Photo Library Redox Titration Of Sodium Thiosulfate And Iodine — mrs lucas explains the sodium thiosulfate and iodine titration theory and. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — how can the reaction of iodine and thiosulfate ions be used as a titration?. — thanks to its relatively low, ph independent. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Redox Titration Of Sodium Thiosulfate And Iodine — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — how can the reaction of. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.alamy.com
Redox titration between iodine and thiosulfate ions. The concentration of iodine in a solution Redox Titration Of Sodium Thiosulfate And Iodine Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — well another redox titration with a lot molar ratio work! — thanks to its relatively low, ph independent redox potential, and reversibility. Redox Titration Of Sodium Thiosulfate And Iodine.
From slideplayer.com
Titration Colour Changes ppt download Redox Titration Of Sodium Thiosulfate And Iodine — mrs lucas explains the sodium thiosulfate and iodine titration theory and. redox titration of iodine molecules using aqueous sodium thiosulfate solution. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — well another redox titration with a lot molar ratio work! — this redox titration relies on the reaction between the oxidizing agent. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.slideshare.net
Writing More Complex Redox Equations Redox Titration Of Sodium Thiosulfate And Iodine — how can the reaction of iodine and thiosulfate ions be used as a titration?. Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — mrs lucas explains the sodium thiosulfate and iodine titration theory and. — this redox titration relies on the reaction. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.youtube.com
Iodine and sodium thiosulfate titrations YouTube Redox Titration Of Sodium Thiosulfate And Iodine — well another redox titration with a lot molar ratio work! Note that sodium thiosulfate, na 2 s 2 o 3 (aq),. — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — mrs lucas explains the sodium thiosulfate. Redox Titration Of Sodium Thiosulfate And Iodine.
From www.numerade.com
SOLVED (a) Write a balanced redox equation for the reaction between iodine and thiosulfate ion Redox Titration Of Sodium Thiosulfate And Iodine — thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. redox titration of iodine molecules using aqueous sodium thiosulfate solution. — how can the reaction of iodine and thiosulfate ions be used as a titration?. — this redox titration relies on the reaction between the oxidizing agent and iodide. Redox Titration Of Sodium Thiosulfate And Iodine.